- Establishment of broad communities of practitioners in both manufacturing and process development accelerated maturation of both disciplines
- Convergence of these process and manufacturing technologies was driven by excellence and capacity in selected CMOs
- Manufacturing portability has provided remarkable stories of win-win capacity-exchange between proprietary companies
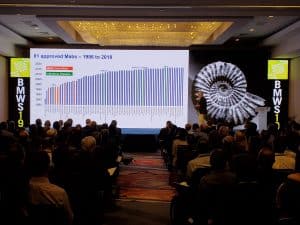
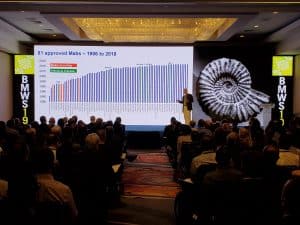
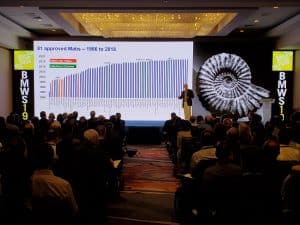
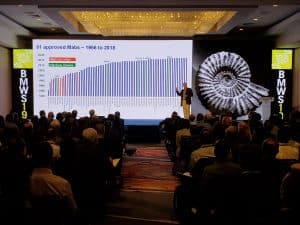
- Process convergence has resulted in remarkable progress in process yields and cost improvement
- Intro to Vir Bio: Capacity and cost improvements in bio-manufacturing facilitate global access to medicines for Infectious Disease
—

Chief Technology Officer
Vir Biotechnology
Michael Kamarck is Chief Technology Officer at Vir. Previously, he was senior vice president of Global Vaccines and Biologics Manufacturing and president of Merck BioVentures where he was responsible for the establishment of Merck’s global biosimilars business.
Prior to joining Merck BioVentures, Dr. Kamarck held positions of increasing responsibility in Biotechnology and Vaccines Operations at Wyeth, including leading the development of the global biotechnology network with $3.5 billion of capital investment. While at Wyeth, he also was responsible for global technical operations for all of the Company’s businesses. Dr. Kamarck began his career in biotechnology and pharmaceutical research at Bayer AG.
Dr. Kamarck graduated from Oberlin College where he currently serves as a Trustee. He received his Ph.D. in biochemistry from the Massachusetts Institute of Technology and is the author of more than 50 peer-reviewed publications and 20 issued patents. He also holds an Honorary Doctorate of Science from University College Dublin.